Detecting minute temperature shifts in the body
The Zadrozny Lab designs molecules that allow magnetic resonance imaging to do things that it currently can’t do.

The noninvasive, life-saving technique known as magnetic resonance imaging works by aligning hydrogen atoms in a strong magnetic field and pulsing radiofrequency waves to convert the response of those atoms into an image.
The field of provenance for MRI, it could be argued, is chemistry – MRI works by exploiting the inherent magnetic properties of individual atoms. What if, instead of just creating images, an MRI machine could extract detailed information about the chemistry of the body – say, the pH levels in the vicinity of a tumor, or the temperature anomalies that occur around an injury? What if the physical principles of magnetic imaging could be applied to all sorts of chemical changes, down to the level of atoms and molecules, and could give us unparalleled new insights into human health and disease?
These “what if” questions drive the work of Department of Chemistry Assistant Professor Joseph Zadrozny and his team of students and researchers. An inorganic chemist who toes the line between chemistry and quantum physics, Zadrozny has built a lab at Colorado State University whose chief goal is to design molecules that allow magnetic resonance imaging to do things that it currently can’t. In doing so, the researchers are uncovering fundamental insights into how the magnetic properties of metal ion-containing molecules respond to their environments, whether that means extremely small shifts in temperature, pH or other metrics.
“We are living, breathing, talking chemical reactors,” Zadrozny said. “If you could image that chemistry, it would be really powerful.”
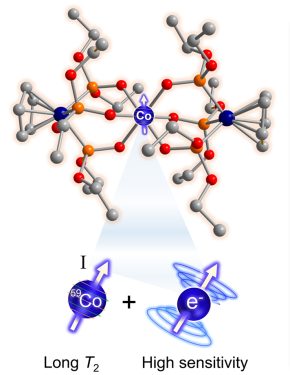
In a breakthrough toward their goal of making new magnetic imaging probes with extreme temperature sensitivity, Zadrozny’s team published a paper in the Journal of the American Chemical Society earlier this year that describes a cobalt-based molecule they’ve engineered to be a noninvasive chemical thermometer. They’ve used their expertise in molecular design to make the cobalt complex’s nuclear spin – a workhorse, fundamental magnetic property – mimic the agile, but less stable sensitivity of an electron’s spin. “Spin” is what gives subatomic particles their magnetism.
By making the cobalt nucleus essentially act like an electron, they’ve shown that this special cobalt complex could someday form the basis for a powerful molecular imaging probe that could read out extremely subtle temperature shifts inside the body.
The imagination could run wild for how this phenomenon could be used: Doctors could detect the minutest temperature shifts around a still-invisible tumor. An in-office thermal ablation procedure could take on molecular-level precision, killing off diseased tissue while avoiding healthy tissue.
Creating a temperature-sensing probe with the cobalt material, which in a doctor’s office might someday be injected or ingested in order to communicate temperature signals from the body, would take advantage of the controllable magnetism of a nucleus. It would also have the desirable property of information readout via radiofrequency waves, which are safe for the human or animal body. Such a magnetic probe would also work at room temperature, the researchers envision.





